Benefits

Rapid Implementation Process
eTMF Master supports the TMF Reference Model by default but lets you customize each study TMF according to its needs as well. Easily set up your study TMF structure via a quick and intuitive process, and enable seamless sponsor-CRO-site collaboration capabilities.

Powerful Content Management
eTMF Master uses Anju’s adaptable enterprise content management technology that is specifically designed for the life sciences industry and provides a range of powerful out-of-the-box capabilities.
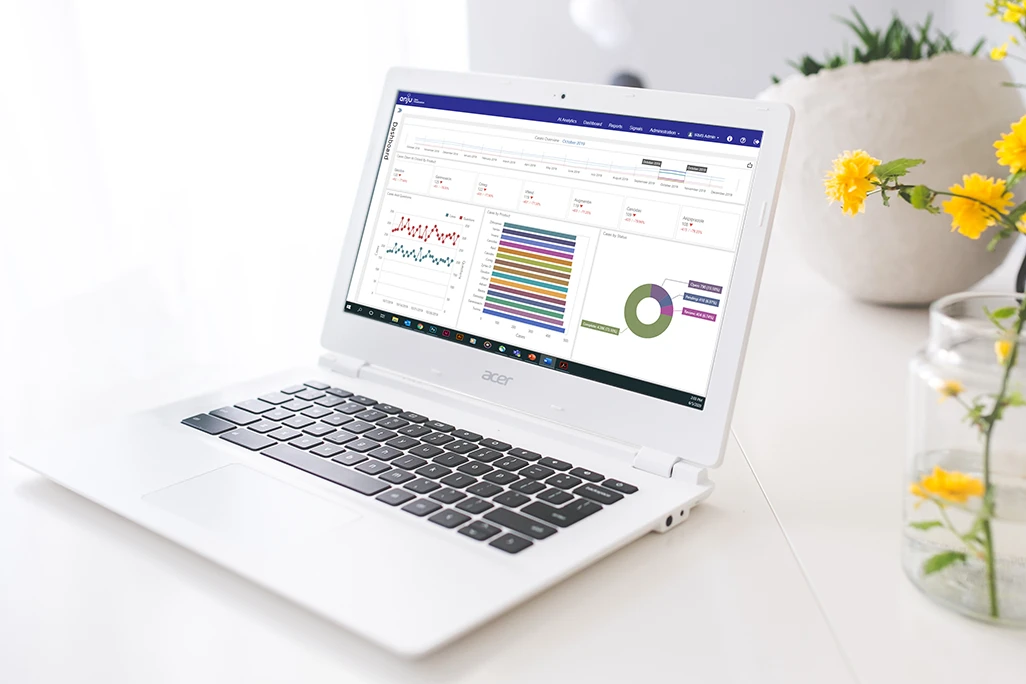
Actionable Insights
eTMF Master’s advanced visualization dashboards provide actionable insights for optimizing the TMF process. You have instant visibility into the health of your TMF via standard metrics, and your TMF is always audit-ready.
Key Capabilities
With eTMF Master, search your TMF at the file, folder, trial or enterprise level. Filter any document list or search results, follow hyperlinks into the content retrieved, and export search results as reports.
eTMF Master provides easy upload and mapping of content into the TMF structure, classification and quality control (QC) review, approval with e-signatures, and such features as moving, printing, and exporting documents from the TMF. Placeholders provide guidance into what documents are expected and help measure the completeness of your TMF.
Use eTMF Master’s workflow management capabilities to ensure that your documents are compliant and that you have what you need to recreate your study if needed. With eTMF Master you can easily manage workflow tasks such as upload, edit, classify, review and approve documents, assign tasks to team members, and send relevant notifications and alerts.
eTMF Master provides a secure, regulatory-compliant environment for storing your TMF. Role-based access can be granted at the client, compound, protocol, country or site levels as well as to specific zones, sections or artifacts.
eTMF Master’s dashboards and reports provide actionable insights into the TMF process. Dashboards are available for tracking and ensuring that your TMF is complete and up-to-date, as well as for metrics on the status of study documents.
eTMF Master is fully compliant with applicable regulations. Among other features, access is fully controlled and monitored, all activities are captured in secure audit logs, and electronic signatures are required and recorded for all document approvals.
Get a Personalized eTMF Demo Today!
Easily manage and gain insights into your TMF process with eTMF Master. Gather all your TMF data into an enterprise content management system with DIA TMF Reference Model mapping and powerful search, workflow and analytics capabilities.
Schedule A Demo



