Benefits
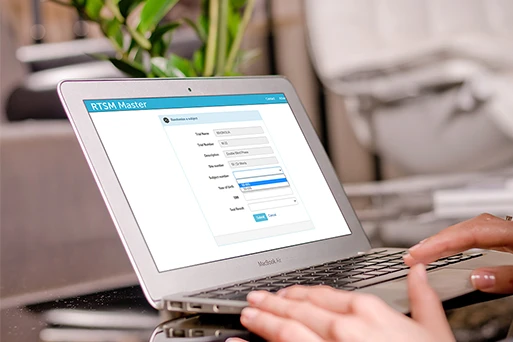
Eliminate Custom Programming
RTSM Master’s unique IRTBuilder workflow feature guides you through the configuration of pre-validated modules to create a user interface and email notifications, in multiple languages, that are specific to your company’s protocol design standards.
Speed Up Trial-by- Trial Implementation
Built-in templates help to speed up trial-by-trial implementation by allowing you to pre-define settings from your preferred configuration.
Key Capabilities
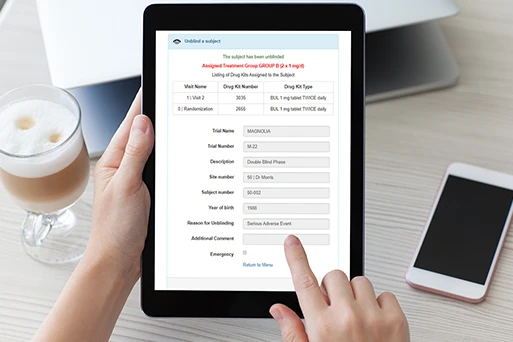
RTSM Master simplifies the management of clinical supplies through an easy-to-navigate system, giving your clinical team scalability to support large studies across the globe without requiring additional resources. The platform’s guided interface takes the complexity out of defining the protocol schedule of drug-dispensing visits. RTSM Master’s feature for automating shipments from multiple warehouses ensures sites have sufficient supplies on hand for their patients. You can manage study medication supplies across multiple depots, including tracking of study drug expiration and restricted kits for use in specific countries. RTSM Master’s Supply Management feature provides detailed tracking of clinical supplies from order generation, shipment to sites, assignment to subjects, return of study medication to sites, and generation of return shipment of kits back to the depot.
RTSM Master easily integrates with TrialMaster, allowing for near real-time, bi-directional integration so data passes effortlessly back and forth, eliminating redundancy in data entry for investigative sites. Additionally, Anju Software’s adaptive eClinical platform allows easy integration of RTSM Master with any 3rd party EDC system.
RTSM Master’s compatibility with multiple pre-validated randomization methodologies (permuted block, dynamic minimization and Zelen) easily accommodates adaptive trial designs. The application’s configurable randomization parameters allow for flexible mid-study changes, including balance changes and opening/closing treatment groups, so site enrollment efforts are not disrupted.
The IRTBuilder Workflow feature guides you through the configuration of pre-validated modules to create a user interface and email notifications in multiple languages that are specific to your company’s protocol design standards. With IRTBuilder, you can eliminate the inefficiencies of custom programming without eliminating the customized user experience.
Any user can access the system in real time from a tablet or phone and perform study-related activities.
Get a Personal Demonstration of RTSM Master Today!
Learn how RTSM Master can speed up your deliverable timelines, reduce costs and eliminate stress!
Schedule A Demo




