Recently, Anju was featured in Drug Development & Delivery with an article about decentralized trials. Read on to learn more on the evolution of decentralized or remote clinical trials, their benefits to sponsors and patients, how technology improves their effectiveness, and how AI accelerates them.
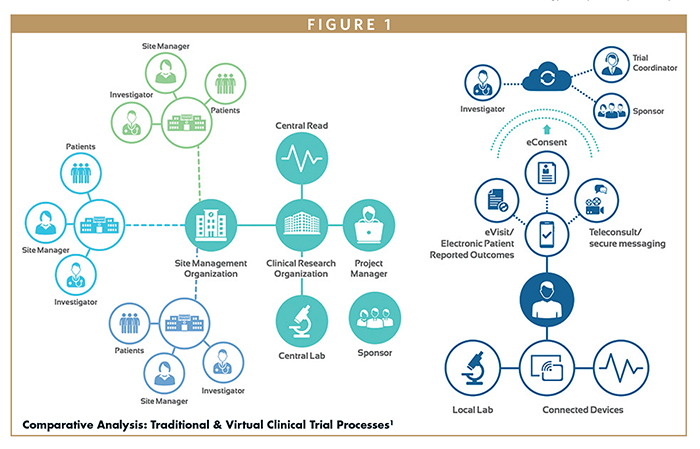
Remote clinical trials are becoming the new standard in clinical research. A variety of terms have been used to describe remote trials that incorporate patient-facing technologies, such as tablets, smartphone apps, or wearable sensors. They have been described as virtual trials, decentralized trials, remote trials, direct-to-patient trials, siteless trials, and hybrid trials. Whereas a digital trial is defined by the method used to capture the clinical trial data, a true digital clinical trial is one in which all data are captured without the use of any paper forms during the conduct of the study.
The COVID-19 pandemic has accelerated the ongoing shift to remote clinical trial monitoring. Remote site access and monitoring platforms are now an essential element of the clinical trial process and a vital connection between the sponsor, the clinical research organization (CRO), and the research site.
Read the full Drug Development & Delivery article here.