As we approach mid 2025, the clinical research landscape reflects resilience, innovation, and global rebalancing. Drawing on TA Scan’s June 2025 analysis, here’s a snapshot of trial volume, structure, disease focus, and emerging dynamics compared to last year and the pre-COVID era.
Clinical Trial Activity: Strong Recovery in 2025
TA Scan captured a total of 6,071 phase I-III interventional trials with a start date in the first half of 2025, marking a 20% increase from the 4,972 trials started in the first 6 months of 2024. This robust rebound brings us back to the activity levels seen in 2021 and exceeding the pre-pandemic levels.
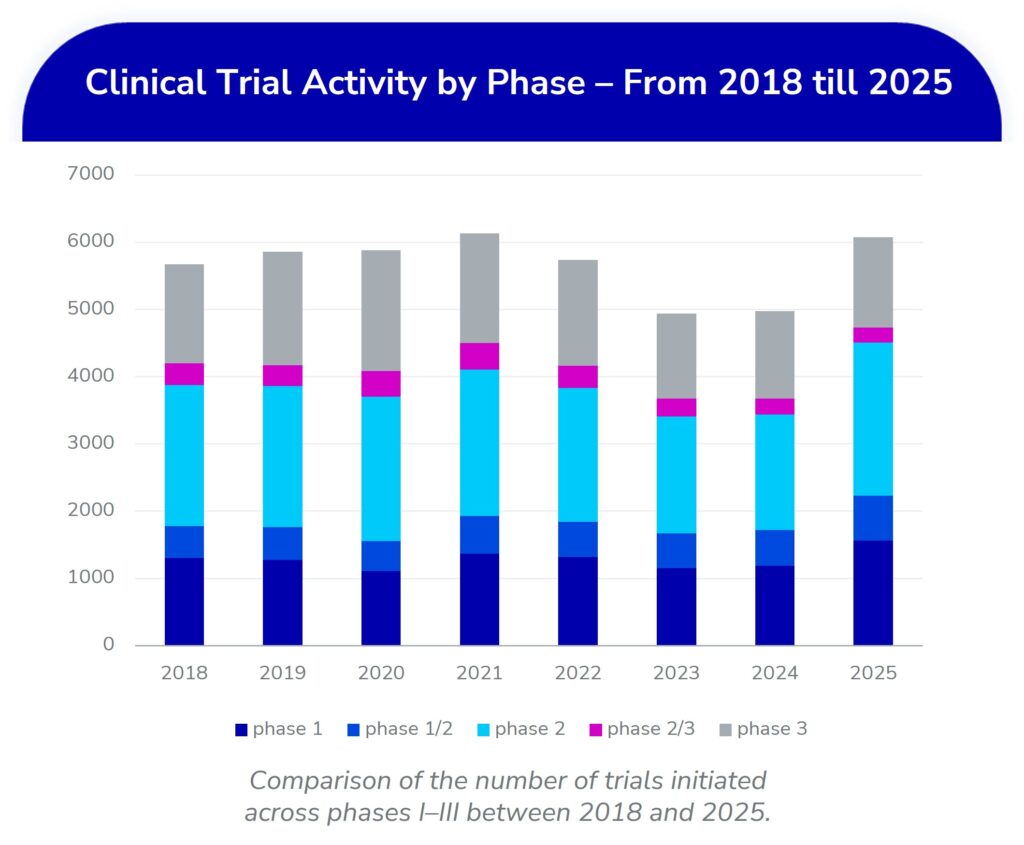
- Phase 2 dominates growth: Phase 2 trials jumped significantly from 1,711 (2024) to 2,278 (2025), the highest level ever seen—making Phase 2 the primary growth engine.
- Phase 1 activity also surged: An increase from 1,187 (2024) to 1,560 (2025), a 21% year-over-year (YoY) increase, suggests a healthy early-stage pipeline.
- Later-phase trials remain stable, though Phase 2/3 studies are decreasing—potentially indicating a shift toward more discrete study phases.
Trial numbers that dipped in the years following the pandemic are now recovering—2025 marks a turning point. Especially early- and mid-phase R&D activity is accelerating, signaling renewed investment across therapeutic stages.
Oncology Still Dominates
TA Scan’s indication-based ranking reveals that the top 10 therapeutic areas are all oncology, with every indication seeing YoY growth—except immune oncology, which decreased slightly from 332 to 323 trials.
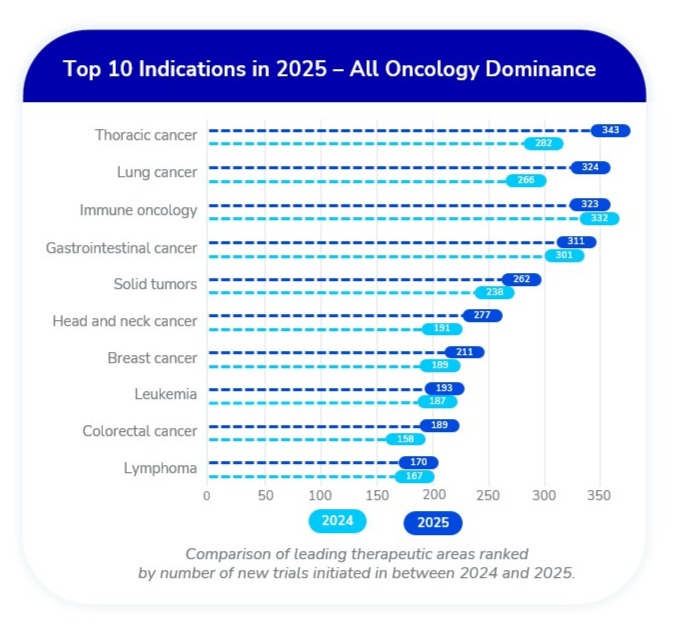
- Fastest growing: Thoracic cancer saw a 25% increase, the highest growth rate among top diseases and now taking the lead.
- Immune oncology saw a slight decline from 332 to 323 trials—the only top-10 oncology area to contract.
- Strong gains were observed in lung, head and neck, breast, and colorectal cancer trials, showing that focus remains strong across all malignancies.
Sponsor Shifts: Changing Priorities Among Top Trial Leaders
Sponsor analysis for 2025 reveals significant shifts in both the key players and their therapeutic focus areas compared to last year (see snapshot of the mid-year 2024 analysis here).
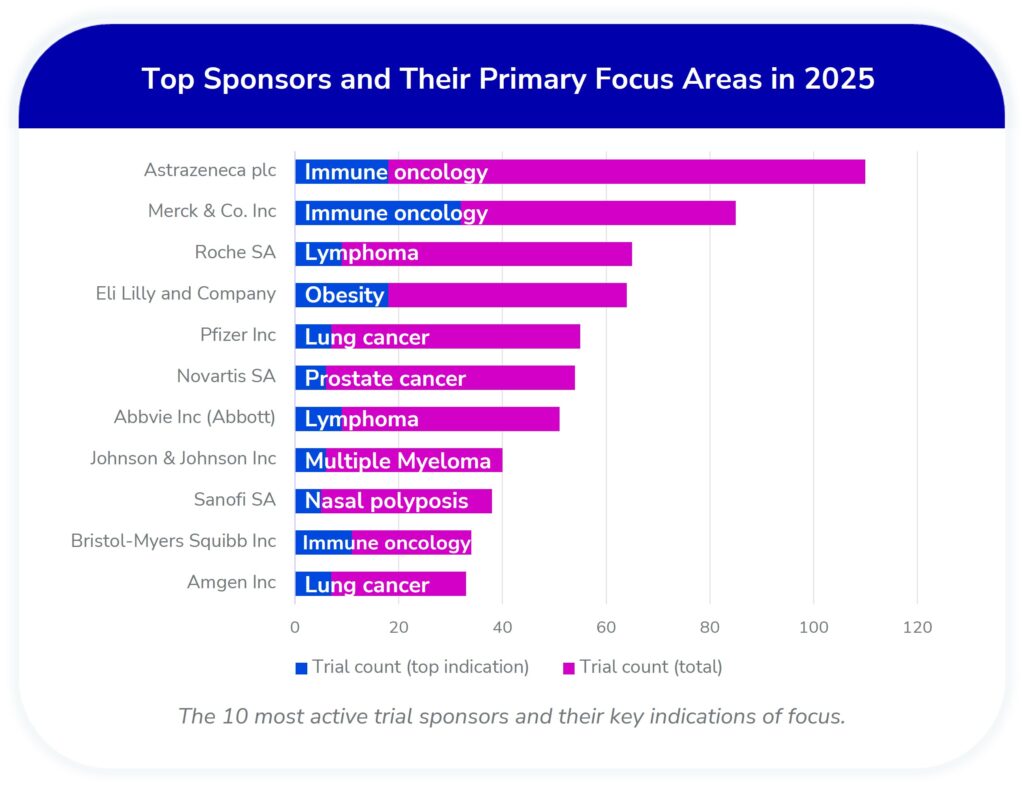
- AstraZeneca has significantly expanded its pipeline, driven by immune oncology, and overtaking peers by total trial volume.
- Merck & Co. holds a strong position (85 trials), maintaining the largest immune oncology portfolio (32 trials).
- Pfizer and Amgen both concentrate on lung and thoracic cancers as strategic priorities.
- Most sponsors continue to prioritize oncology—though often with different lead indications than last year—while Sanofi (nasal polyposis) and Eli Lilly (obesity) stand out for diversifying beyond cancer.
- New entrants in the top 10: Amgen and Bristol-Myers Squibb, while Boehringer Ingelheim and GSK have dropped out.
Global Distribution: North America and Europe Lead Trial Activity
Regional analysis underscores the continued concentration of clinical trial activity in North America (2,134 trials) and Europe (1,488 trials), which together account for nearly half of all studies initiated. East Asia, driven largely by China, also remains a critical hub with 1,268 trials.
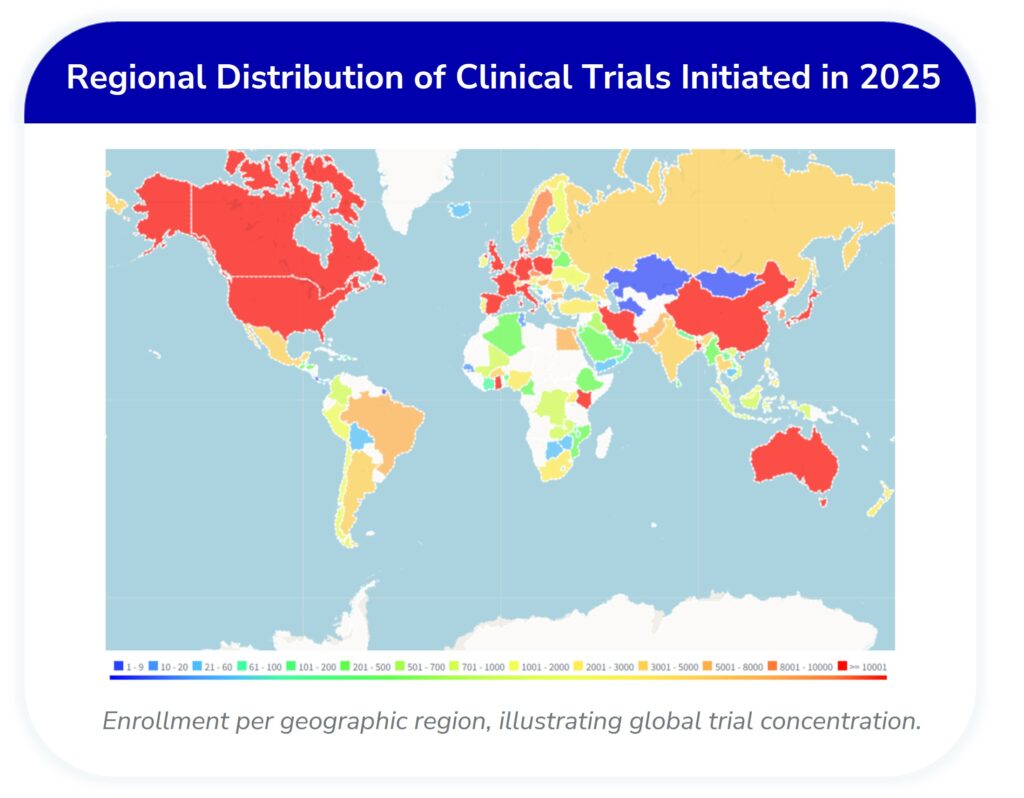
- North America: 2,134 trials—the single largest share globally.
- Europe: 1,488 trials, reinforcing its role as a key region for research.
- East Asia (China): 1,268 trials, reflecting strong regional investment.
The geographic spread highlights both entrenched centers of innovation and emerging regions with increasing clinical research activity.
Looking Ahead
The 2025 clinical research landscape is defined by both resurgence and reinvention. Early- and mid-phase trials are gaining momentum as sponsors intensify discovery efforts across oncology, obesity, and emerging indications. At the same time, rising trial complexity—from adaptive protocols to master frameworks—demands more sophisticated planning and operational agility.
Equally critical is the continued push to improve patient inclusion and diversity. With regulatory guidance, public accountability, and scientific necessity all converging, sponsors can no longer afford to treat diversity as an afterthought. Incorporating representative populations is essential for generating credible, generalizable evidence—especially in areas like immuno-oncology, where precision medicine depends on robust, inclusive datasets.
As we look ahead, successful organizations will be those that embrace innovation across trial design, patient engagement, and execution. Navigating these shifts requires not only strategy but also the right technology. Clinical trial intelligence platforms like TA Scan enable teams to anticipate trends, identify the best sites and investigators, and analyze predictive feasibility simulations that support faster, more informed decision-making.
Stay tuned for our year-end analysis, where we’ll explore how sponsors are accelerating site selection, strengthening feasibility strategies, and charting the next wave of therapeutic breakthroughs.
Images used under license by https://stock.adobe.com/
Authored by Elke Ydens, Associate Director of Business Solutions, Data Division
Elke Ydens, Associate Director of Business Solutions at Anju’s Data Division, brings over a decade of life sciences experience and a PhD in Biochemistry and Biotechnology from the University of Antwerp. As a Subject Matter Expert in Data Science, she adeptly addresses customer needs, leveraging her background in neuro-immunology and biochemistry. Elke remains dedicated to professional growth, contributing to industry publications, and staying updated on industry trends, while also finding success in extracurricular pursuits, formerly competing in world and European bridge championships, and more recently active in beekeeping and coaching. Connect with Elke on LinkedIn to explore her achievements further.
