Joined by clinical operation teams from around the globe in Barcelona, Spain, Anju Software took center stage at the SCOPE (Summit for Clinical Ops Executives) Europe 2023 conference this week.
For the second time this year, Anju’s TA Scan won the attendee-voted Best of Show Award for its expanded European diversity and demographic data. This accolade serves as a testament to the unwavering dedication and brilliance of the team, reaffirming Anju’s steadfast commitment to delivering groundbreaking life science solutions that leave a lasting impact.
The spotlight at SCOPE Europe 2023 shone brightly on Anju Software’s TA Scan as it emerged victorious, standing out from seven other exceptional product finalists that graced the exhibit hall floor. However, it wasn’t just the competition that set Anju apart; it was the resounding endorsement of the community that made all the difference.
Over 500 attendees at the Summit for Clinical Ops Executives had the privilege of evaluating these innovations in person. Their unanimous choice was clear: Anju’s TA Scan diversity data and analytics.
Health Equity Challenges in Clinical Trials
Achieving equitable patient representation in clinical trials has proven to be a challenging endeavor. Drug development plans necessitate a comprehensive examination of incidence and prevalence in various racial, ethnic, and socio-economic subgroups, as these factors significantly vary across disease areas. Understanding inter-ethnic differences in drug responses requires meticulous analysis. To address this issue, sponsors are now mandated to submit Diversity Action Plans (DAPs) to the FDA for Phase III and pivotal studies. However, the critical challenge lies in the lack of robust and reliable global data on race, ethnicity, and socio-economic characteristics, which hinders the identification and inclusion of relevant population groups. While the United States discloses public data on these factors, Europe faces considerable variability in healthcare systems, policies, and data protection regulations, leading to a lack of consistent data collection. Some European countries collect significant data, such as the United Kingdom, the Netherlands, and Finland, while others like France, Germany, and Sweden share little to no data due to concerns related to discrimination, individual privacy, or historical sensitivities.
Data strategies that ensure the inclusion of racially and ethnically diverse participants in clinical trials have become increasingly critical. Without adequate data, inequities in healthcare remain unnoticed and unaddressed. The challenge is amplified by the varying data availability and privacy regulations in different European countries, which creates a complex landscape for sponsors and regulators striving to address these issues. To enhance patient representation and tackle disparities effectively, a concerted effort is required to standardize data collection, establish a global framework, and address the concerns of individual privacy and discrimination in data gathering while promoting transparency and equity in clinical trials across various regions.
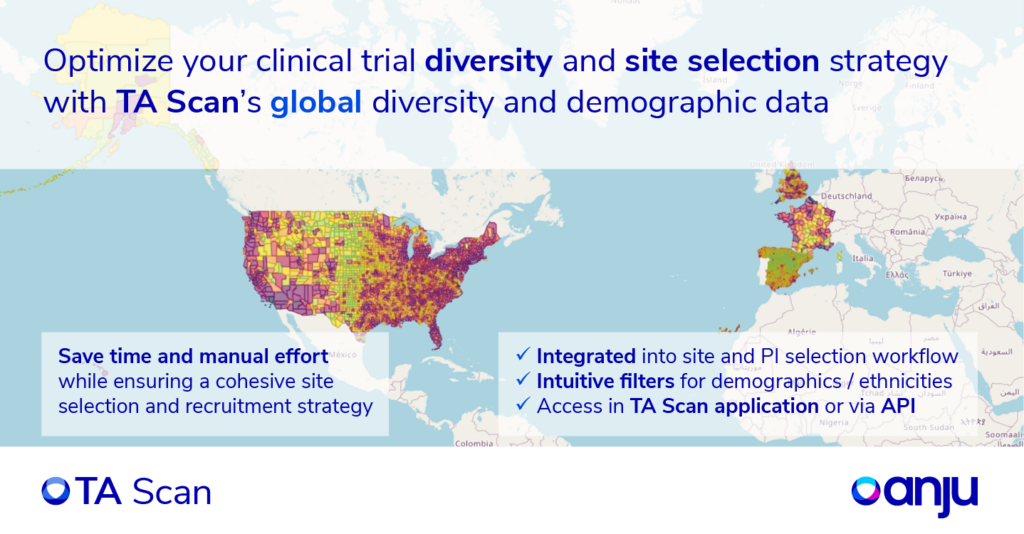
Anju’s Clinical Intelligence Solution
TA Scan, Anju’s clinical and commercial business intelligence solution, is at the forefront of addressing the critical challenges in diversity data for clinical trials. This versatile platform empowers clinical research professionals with its innovative features and comprehensive capabilities, offering a holistic view of essential data on a single map. This data includes ethnicity, socio-economic information, and site/investigator experience. The streamlined approach of TA Scan significantly accelerates site selection and recruitment strategies, reducing both time and manual effort in the clinical research process.
Users can easily filter, visualize, and analyze this data within the TA Scan application, export it for in-depth analysis, or access it through API Services. Anju’s Data-as-a-Service offering also provides sponsors and contract research organizations (CROs) with invaluable insights to enhance feasibility assessments, site/investigator/KOL identification, and diversity strategies.
One distinctive feature of TA Scan is its capacity to visualize and analyze newly integrated European diversity data besides US diversity data. Anju recognizes the importance of representing diverse patient populations in clinical trials, aiming to facilitate this moral obligation by enabling customers to gather comprehensive and representative data. This commitment ensures that clinical research remains a robust and representative process, promoting inclusivity and accessibility.
As a trusted technology solutions partner in the life sciences industry, Anju’s TA Scan continues to drive the pursuit of medical breakthroughs through data accessibility, inclusivity, and regulatory excellence from concept to cure.
Innovative Data Science Technology
The TA Scan technology excels in the collection and aggregation of diverse and unstandardized data sources at a large scale. Their patented TrialCloud technology facilitates the semantic linkage of various data types, unveiling concealed insights. Recent enhancements have been made to their processes and product, introducing an additional data type to complement TA Scan’s meticulously curated data within a unified repository. The intricate linkage of diversity data with geographical units guarantees precision and granularity, while their technology supports data visualizations, enhancing the decision-making process.
The implementation of this new data processing approach expands the platform’s capabilities to include data from numerous countries. Anju and TA Scan maintain their unwavering dedication to continuous improvement and innovation, with a focus on enabling the extraction of global insights that promote equity in clinical trials and greater inclusivity in the field of clinical research.
Take Your Trials to The Next Level
Today, we invite you to take the next step in your clinical trials journey. Contact us to discover how Anju’s TA Scan can revolutionize your approach and leave a lasting impact in the field of life sciences. Together, let’s pave the way toward a more inclusive and accessible future for all patients.
